 |
The Edwin Surgical Papyrus, which was written in ancient Egypt in the 17th century B.C., already described about spinal cord injury and states that it is “untreatable”. Even in modern times, this hopeless situation is still the same, with 5,000 new injuries per year in Japan, and patients suffer from severe motor and sensory paralysis. The essence of this condition is the severing of neuronal axons and their inability to regenerate. Neuronal axons are long neurites and are primarily responsible for information output in neural circuits. In humans, the longest axons are approximately one meter long. The spinal cord is a bundle of these axons, and when damaged by trauma or other causes, these axons are severed, resulting in a breakdown of the neural circuitry. Although many of the damaged neurons themselves survive, they are not able to regrow their axons to reorganize the neuronal circuit.
Santiago Ramón y Cajal, a Spanish neuroanatomist active in the 19th and 20th centuries, observed injured spinal cords and found that the tips of damaged axons had an abnormal swollen structure that contained vacuoles, which he named as the dystrophic endball. He hypothesized that dystrophic endball was a responsible structure for inability of axonal regeneration in the central nervous systems.
In the late 1900s, Aguayo et al. bypassed the central and peripheral sides of the injured spinal cord with peripheral nerve bundles as grafts, and found that the axons of the central nervous systems regenerated within these grafts. This led researchers to believe that the failure of axonal regeneration in the central nervous systems was due to the environment of the central nervous systems rather than to the nature of the neuronal cells themselves. In fact, a number of "axon regeneration inhibitors" were identified. Chondroitin sulfate is one such factor. Chondroitin sulfate is produced by the glial scar at the lesion and strongly inhibits axonal regrowth. In rodent models of spinal cord injury, enzymatic digestion of chondroitin sulfate in the injured area by chondroitinase ABC, a degrading enzyme for chondroitin sulfate, leads to promote recovery of motor function after injury (1). Importantly, extracellular chondroitin sulfate transforms the tip of axons into dystrophic endballs in vitro (2). Furthermore, protein tyrosine phosphatase σ (PTPσ), which belongs to a receptor-type tyrosine phosphatase, was identified as a chondroitin sulfate receptor in axons (3). Interestingly, PTPσ had been already known as a receptor for heparan sulfate, a glycosaminoglycan together with chondroitin sulfate, and is dimerized and inactivated by heparan sulfate. Contrary, when chondroitin sulfate acts as a ligand, it is monomerized and activated (4). However, the cellular mechanism of dystrophic endball formation and the substrate which is dephosphorylated by PTPσ have long been unclear.
The authors found an abnormal accumulation of autophagosomes in the dystrophic endball. Autophagosomes fuse with lysosomes to form autolysosomes, but the fusion of autophagosomes with lysosomes was strongly inhibited in dystrophic endball (4). On the other hand, artificial inhibition of autolysosome formation by lysosome inhibitors or by knockdown of SNARE protein, which is essential for autophagosome-lysosome fusion, transformed healthy axon to dystrophic endball-like abnormal structures. These findings indicate that inhibition of autolysosome formation is a sufficient and needed phenomenon for dystrophic endball formation (5) (Figure).
As described above, chondroitin sulfate was predicted to monomerize PTPσ as a receptor and activate its intracellular tyrosine phosphatase domain, but its substrate was unknown (4). Based on the aforementioned dystrophic endball phenotype, the authors focused on molecules involved in autophagosome-lysosome fusion and identified cortactin as a substrate of PTPσ (5). Cortactin is recruited to the lysosomal surface when the tyrosine at 421 is phosphorylated by tyrosine kinases such as src, where it stabilizes actin fibers and provides the scaffold necessary for autophagosome-lysosome fusion. PTPσ directly dephosphorylates phosphorylated cortactin, and thus, inhibits the fusion of autophagosomes and lysosomes. In fact, cortactin was predominantly dephosphorylated in dystrophic endball compared to healthy axonal tips. Knockdown of cortactin also induced dystrophic endball in healthy axon terminals. This indicates that cortactin is responsible for the induction of dystrophic endballs downstream of PTPσ (Figure).
Recently, axonal spheroids were shown to be directly induced by Aβ plaques in Alzheimer's disease and to inhibit neural network conduction (6). The abnormal accumulation of autophagosomes and multivesicular bodies (MVBs) in this spheroid was also identified. Further studies will be needed to reveal the similarities and differences with the dystrophic endballs.
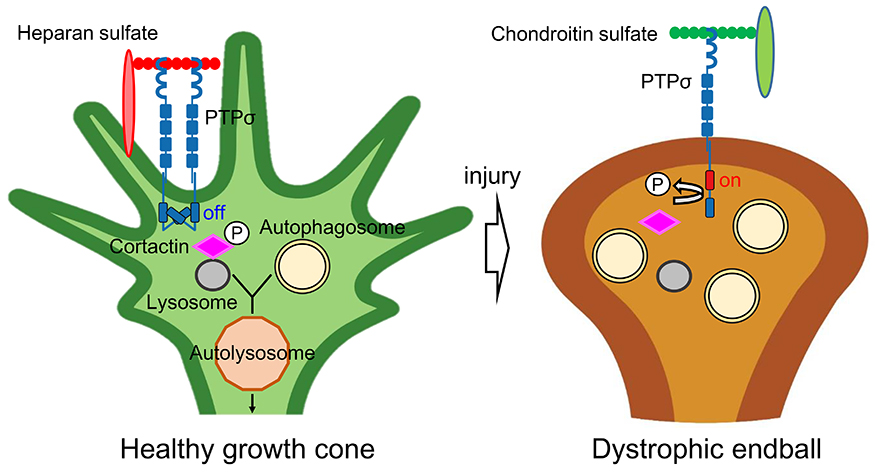
Figure
In healthy growth cones (left), heparan sulfate multimerizes and inactivates PTPσ. In this situation, autophagy at the axon tip proceeds smoothly and the axon elongates. In CNS injury (right), glial scar-derived chondroitin sulfate monomerizes and activates PTPσ, which dephosphorylates cortactin and inhibits fusion between autophagosome and lysosome. As a result, autophagosomes abnormally accumulate at the axon tip, and dystrophic endballs are induced.
|
Kazuma Sakamoto
(Nagoya University, Institute for Glyco-core Research (iGCORE))
| References |
| (1) |
Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB: Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640, 2002 |
| (2) |
Tom VJ, Steinmetz MP, Miller JH, Doller CM, Silver J: Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci. 24, 6531–6539, 2004 |
| (3) |
Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG: PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326, 592–596, 2009 |
| (4) |
Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, Gallagher JT, Jones EY, Flanagan JG, Aricescu AR: Proteoglycan-specific molecular switch for RPTPσ clustering and neuronal extension. Science 332, 484-488, 2011 |
| (5) |
Sakamoto K, Ozaki T, Ko YC, Tsai CF, Gong Y, Morozumi M, Ishikawa Y, Uchimura K, Nadanaka S, Kitagawa H, Zulueta MML, Bandaru A, Tamura JI, Hung SC, Kadomatsu K: Glycan sulfation patterns define autophagy flux at axon tip via PTPRσ-cortactin axis. Nat. Chem. Biol. 15, 699–709, 2019 |
| (6) |
Yuan P, Zhang M, Tong L, Morse TM, McDougal RA, Ding H, Chan D, Cai Y, Grutzendler J: PLD3 affects axonal spheroids and network defects in Alzheimer’s disease. Nature 612, 328–337, 2022 |
Jun. 15, 2023
|
|---|







