 |
Aggrecan (Acan) is a typical large chondroitin sulfate (CS) proteoglycan (PG) of the extracellular matrix (ECM) type, which is abundant in cartilage. Acan (1) has a gene family, which includes versican (Vcan) (2), neurocan, and brevican. This family is also termed lectican family, as either contains a lectican domain. Core proteins of the Acan family have globular domains G1 and G3 at the N- and C-termini, respectively, and chondroitin sulfate (CS)-binding domains in the middle (Fig. 1). Acan has another globular domain called G2. The G1 domain consists of three subdomains, A, B, and B', and specifically binds to hyaluronan (HA). Cartilage link protein (Crtl1, HA-Proteoglycan Link Protein-1, HAPLN-1), another molecule homologous to the G1-domain, binds specifically to the G1 domains of Acan and Vcan as well as HA. The link protein also has four family members. An Acan family PG, a HAPLN member, and HA interact with each other and form a stable complex termed "PG aggregates, which accumulate in the ECM (Fig. 1)." Currently, the information on genetically modified mice is posted on the Mouse Genome Informatics (MGI) website (https://www.informatics.jax.org/). In this article, I describe gene mutant mice of Acan and Vcan, and their phenotypes.
There are two strains of spontaneous Acan gene mutant mice, i.e., cartilage matrix deficiency (cmd) and cmd-bc. In cmd, a 7-bp deletion in exon 5 encoding the B subdomain of the G1 domain causes a frameshift and premature termination. In cmd-bc, a deletion spanning from intron 1 and downstream of 3’-untranslated region removes exon 2-18. In cmd/cmd, the columnar structure of growth plate chondrocytes is disturbed, and endochondral ossification is impaired, resulting in dwarfism and death immediately after birth. Cmd/+ develops slight dwarfism as well as curvature of the spine. Cartilage link protein (HAPLN-1)-deficient mice exhibit a reduced Acan deposition to ~25% in cartilage and mild phenotypes similar to cmd/cmd, indicating that HAPLN-1 contributes to the formation and maintenance of stable PG aggregates in vivo. Acan is present in the perineuronal net (PNN) of the brain. In mice with brain-specific deletion of Acan expression, disruption of PNN structure and enhancement of neuronal plasticity are observed, suggesting that Acan is essential for PNN structure and function (3).
Vcan has two characteristic expression patterns. In adult tissues, it is constitutively expressed, serving as a structural macromolecule of the ECM. In embryos, it is transiently expressed and forms a provisional matrix. Vcan rapidly disappears while authentic ECM molecules accumulate in the ECM. Transiently expressed Vcan forms a space locally where cells migrate and differentiate. The provisional matrix is replaced with the authentic ECM generated by the differentiated cells.
The first Vcan gene mutant mice were generated by the gene trap method, and termed hdf. The homozygotes (hdf/hdf) exhibit severe cardiac malformation and die at embryonic day 10.5 (E10.5). VcanΔ3/Δ3 mice, generated by inserting a neomycin-resistant gene into exon 3 encoding the A subdomain of the G1 domain, express lower levels of Vcan lacking the A subdomain than wild-type mice. Whereas VcanΔ3/Δ3 mice with C57Bl/6 background show cardiac malformation similar to hdf/hdf and die at E10.5, those with mixed backgrounds with Balb/c survive up to the neonatal period with ventricular septal defect (VSD) (4). Hdf/+ mice also develop VSD, suggesting that Vcan, exhibiting dynamic expression patterns during cardiogenesis, plays a unique function at each region at each step of the process. Vcan is transiently expressed in mesenchymal condensation areas of cartilage primordium and therefore speculated to play an important role in cartilage development. Prx1-Vcan mice lacking Vcan expression at the mesenchymal condensation areas exhibit delayed chondrocyte differentiation and impaired joint formation by altered TGFβ signaling, but cartilage tissue is formed.
During extracellular matrix transformation, such as inflammation and tumor invasion, Vcan is highly expressed in fibroblasts and infiltrating macrophages. Implantation of tumor cells mixed with Cre-expressing adenovirus into the subcutaneous tissue of Vcanflox/flox mice resulted in the loss of Vcan expression in the host stromal cells of the mice, along with collagen fiber weakening and tumor cell proliferation. Although many research results have shown a positive correlation between tumor Vcan expression and malignancy, host stromal Vcan suppresses tumor growth by maintaining the ECM structure. There are four major splice variants of Vcan (Fig. 2). While transiently expressed Vcan is V0 or V1, V2 is constitutively expressed in the brain. Vcan (tm1Zim) mice, created to delete V0 and V2 expression, exhibit structural changes of Ranvier's nodules, including reduced deposition of tenascin-R and phosphacan in the ECM (5).
Elucidation of the function of Acan in cartilage and the brain leads to the development of cartilage bioengineering and improving brain function. Vcan regulates the ECM transformation in inflammation and tumor invasion. Elucidation of its function in the ECM construction and destruction will lead to the treatment of these pathologic conditions (6).
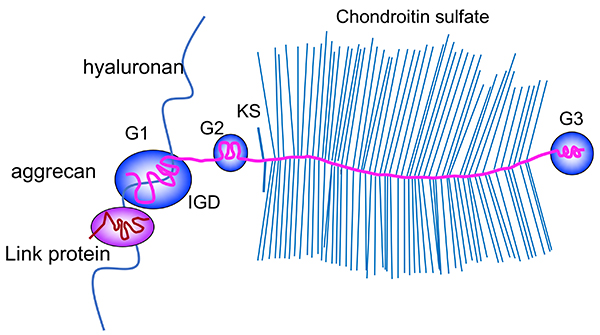
Figure 1. Structure of Acan aggregates.
Aggrecan (Acan) core protein consists of three globular domains, G1, G2, and G3, two glycosaminoglycan attachment domains, i.e., keratan sulfate (KS) domain and chondroitin sulfate (CS) domain, and an interglobular domain between G1 and G2. The N-terminal G1 domain binds to hyaluronan, and the binding is stabilized by another molecule homologous to the G1, termed cartilage link protein (hyaluronan and proteoglycan binding link protein-1, HAPLN-1). More than one hundred CS chains are attached to the Acan core protein and contribute to water retention.
|
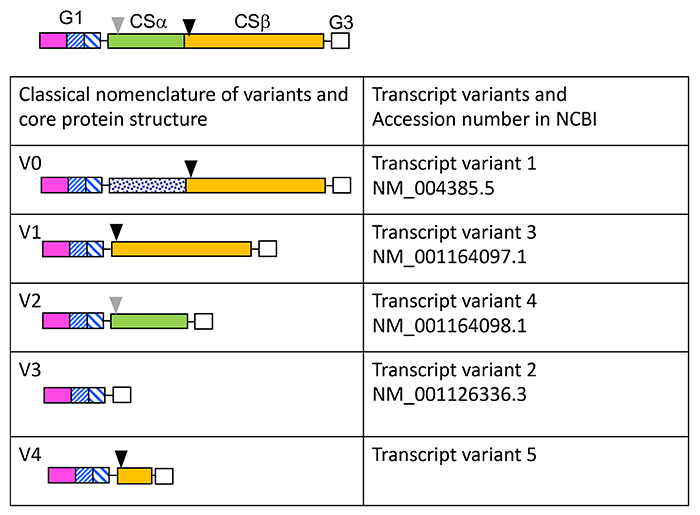
Figure 2. Transcriptional variants of Vcan.
Vcan core protein structure is shown with the G1, G3, and two chondroitin sulfate-binding domains, i.e., CSα (green color) and CSβ (mustard color). Solid and grey arrowheads indicate initial and alternative cleavage sites by ADAMTS versicanases. Classical variant forms were termed V0, V1, V2, and V3 (left column), which correspond to variant forms 1, 3, 4, and 2 in the National Center for Biotechnology and Information (NCBI). Recently, another variant form was reported (7), which is not on the NCBI yet.
|
Watanabe Hideto
(Institute for Molecular Science of Medicine, Aichi Medical University)
| References |
| (1) |
Watanabe H, Yamada Y, Kimata K: Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J. Biochem. 124, 687-693, 1998 |
| (2) |
Islam S, Watanabe H: Versican: A dynamic regulator of the extracellular matrix. J. Histochem. Cytochem. 68, 763-775, 2020 |
| (3) |
Rowlands D, Lensjo KK, Dinh T, Yang S, Andrews MR, Hafting T, Fyhn M, Fawcett JW, Dick G: Aggrecan directs extracellular matrix-mediated neuronal plasticity. J. Neurosci. 38, 10102-10113, 2018 |
| (4) |
Hatano S, Kimata K, Hiraiwa N, Kusakabe M, Isogai Z, Adachi E, Shinomura T, Watanabe H: Versican/PG-M is essential for ventricular septal formation subsequent to cardiac atrioventricular cushion development. Glycobiology 22, 1268-1277, 2012 |
| (5) |
Dours-Zimmermann MT, Maurer K, Rauch U, Stoffel W, Fassler R, Zimmermann DR: Versican V2 assembles the extracellular matrix surrounding the nodes of ranvier in the CNS. J. Neurosci. 29, 7731-7742, 2009 |
| (6) |
Watanabe H: Aggrecan and versican: two brothers close or apart. Am. J. Physiol. Cell Physiol. 322, C967-C976, 2022 |
| (7) |
Kischel P, Waltregny D, Dumont B, Turtoi A, Greffe Y, Kirsch S, De Pauw E, Castronovo V: Versican overexpression in human breast cancer lesions: known and new isoforms for stromal tumor targeting. Int. J. Cancer 126, 640-650, 2010 |
Jun 15, 2023
|
|---|








