| Regioselective Desulfation of Heparin | |
|
 |
Although heparin is polydisperse in the covalent structure, its backbone mainly (typically more than 90%) consists of alternating disaccharide repeats of 1,4-linked D-glucosamine and 1,4-linked L-iduronate residues. This molecule carries many sulfate groups to bind a variety of proteins, exerting various biological activities of heparin such as anti-coagulant activity, cell proliferation activity, etc [1]. Since negative charges of the sulfate groups are an important driving force for the heparin-protein interactions, their introduction (sulfation) and removal (desulfation) at specific positions may alter the spectrum of the heparin activities and can result model compounds that explain the effects of the individual sulfate groups on the molecular recognition. The three main sulfate groups of heparin, those linked to O-2 of the iduronate residue (2-O-sulfate), O-6 of the iduronate residue (6-O-sulfate) and amino group (2-N) of the glucosamine residue (N-sulfate) are selectively removed by relatively convenient chemical methods such as those described bellow, and these methods are applied either solely or jointly to obtain the polysaccharide derivative with an appropriate sulfation pattern (Figure). | |
|
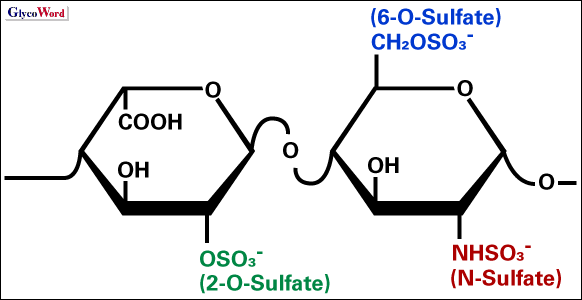 |
Fig. Main repeating structure of heparin
de-N-selfation: solvolitic reaction
de-2-O-sulfation: alkali-mediated reaction
de-6-O-sulfation: silylating reagent-mediated reaction |
|
|
|
De-N-sulfation: Both O- and N-sulfate groups can be removed by a “solvolytic” reaction, when pyridinium salt of heparin is heated at 80°C for four hours in dimethylsulfoxide [2]. A small amount of methanol or water added to the reaction system (ca. 10% v/v) as an acceptor of liberated sulfate groups improves the efficiency of the reaction. Since the elimination rate of the N-sulfate group is much greater than that of the O-sulfate group, the solvolytic reaction under mild conditions (reaction at 20°C or below) causes sufficiently selective de-N-sulfation [3]. The free amino groups liberated after de-N-sulfation are usually acetylated (N-acetylation), as their positive charge may interact and/or interfere with the negative charge of O-sulfate groups.
De-2-O-sulfation: Sulfate groups may be eliminated to form etheric linkages under strongly alkaline conditions, when a sulfate group and a free hydroxyl group are suitably arranged in the three-dimensional structure. Heparin, which has a sulfate group at O-2 and a free hydroxyl group at C-3 in the trans configuration in the iduronate residues, undergoes elimination of the 2-O-sulfate groups and simultaneous formation of 2,3-epoxide rings, when its aqueous solution is alkalified and freeze-dried. The epoxide rings are cleaved to yield couples of adjacent hydroxyl groups. In this step, there may be two routes leading either to the L-galacto or L-ido configuration of the recovered hydroxyl groups. However, the latter route is preferred to result in de-2-O-desulfation without change in the configuration of iduronate residues, when heparin in sodium hydroxide solution of more than 0.2 M is freeze-dried and the product is simply dissolved in water and dialyzed [4].
De-6-O-sulfation: Since the elimination rate of sulfate groups of heparin in solvolytic desulfation follows the order N-sulfate >>> 6-O-sulfate > 2-O-sulfate, the former two sulfate groups are preferentially removed solvolytically under appropriate conditions. The subsequent N-resulfation yields preferentially de-6-O-sulfated heparin [5]. As the reaction rate of 6-O-sulfate is close to that of 2-O-sulfate, however, this method may cause incomplete de-6-O-sulfation and substantial de-2-O-sulfation. An alternative de-6-O-sulfation method employs a silylating reagent, N-methyl-N-(trimethylsilyl)trifluoroacetamide (MTSTFA) [6]. Briefly, pyridinium salt of heparin soaked in pyridine is mixed with MTSTFA, and heated at 110°C for two hours. During this step, sulfate groups linked to primary hydroxyl groups such as O-6 are eliminated while those at other positions are conserved. Trimethylsilyl groups introduced to hydroxyl groups (both those originally occurring in the starting material and those newly derived from 6-O-sulfoxyl groups) are removed when the reaction mixture is mixed with water and dialyzed. |
|
|
|
|
| Takano Ryo (University of the Ryukyus, Okinawa National College of Technology)
| |
|
|
| References |
(1) |
For review, HE Conrad, Heparin binding proteins, Academic Press, New York, 1998.
|
|
(2) |
K Nagasawa, Y Inoue, T Kamata, Carbohydr. Res. 58, 47-55, 1977 |
|
(3) |
Y Inoue, K Nagasawa, Carbohydr. Res. 46, 87-95, 1976 |
|
(4) |
M Jaseja, RN Rej, F Sauriol, AS Perlin, Can. J. Chem. 67, 1449-1456, 1989
|
|
(5) |
H Baumann, H Scheen, B Huppertz, R Keller, Carbohydr. Res. 308, 381-388,
|
|
(6) |
R Takano, Z Ye, T-V Ta, K Kamei, Y Kariya, S Hara, Carbohydr. Lett. 3, 71-77, 1998
|
| |
|
|
|
| Jun. 15, 2001 |
|
| |
|
|
|
|



