|
Syndecans and Glypicans - Cell surface proteoglycans |
 |
|
 |
The syndecan family contains four members (syndecan-1/syndecan,
syndecan-2/fibroglycan, syndecan-3/N-syndecan, syndecan-4/ryudocan
(amphyglycan)), which are transmembrane heparan sulfate proteoglycans
(HSPGs)(1, 2). These HSPGs exhibit cell type-specific distribution
with vascular endothelial cells expressing syndecan-1, -2, and
-4, and predominant targeting to basolateral surfaces. The syndecan
family members are type I integral membrane proteins with homologous
transmembrane and cytoplasmic domains. The combined transmembrane/cytoplasmic
domains contain four well-conserved tyrosine residues, which might
serve important roles for biological function.
The cytoplasmic tail of syndecan-1 interacts with intracellular
microfilaments, and that of syndecan-4 with focal adhesion molecules.
Syndecan-1 has both heparan sulfate and chondroitin sulfate GAG(glycosaminoglycan)
chains with a tissue-specific structural polymorphism due to distinct
post translational modifications. Syndecan-1 has also been purified
as an anticoagulant HSPG from endothelial cells or as a bFGF receptor
molecule in golden hamsters. Thus, this polymorphism of syndecan-1
likely reflects distinct HSPG functions.
The glypican, another cell surface HSPG family, is composed of
five members (glypican-1/glypican, glypican-2/cerebroglycan, glypican/OCI-5,
glypican-4/K-glypican, glypican-5)(2, 3). Glypican family members
possess an extracellular region with GAG attachment sites, 14
invariant cysteine residues, which stabilize a highly compact
tertiary structure, and a COOH-terminal GPI(glycosylphosphatidylinositol)
anchor. Glypican family members are selectively expressed on different
cell types with only glypican-1 present on vascular endothelial
cells. These HSPGs are mainly targeted to apical surfaces, and
this process is partially dependent upon the extent of glycanation.
It is also suggested that glypican plays an important role in
regulating the biological activity of fibroblast growth factors
via HS GAG chains like syndecan. |
 |
|
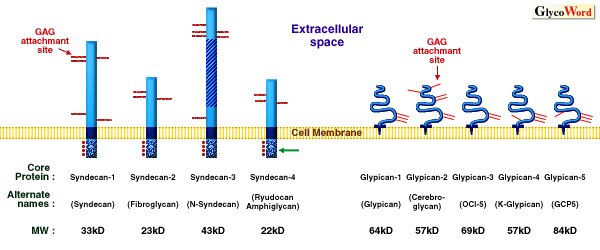 |
Figure Legend
Cell surface HSPG familiesF
The membrane spanning syndecans and GPI anchored glypicans. Potential
and identified GAG attachment sites are indicated by red lines.
For the syndecans, the homologous transmembrane domain (dark blue)
and intracellular domain (stipple) with conserved tyrosines (dots),
as well as the Thr, Ser, Pro rich domain of syndecan-3 (crosshatch)
are indicated. For the glypicans, indicated are the core protein
(blue solid line) and GPI anchor (dark blue part). (Modified from
ref. 2) |
|
|
|
Tetsuhito Kojima (Nagoya University, School of Medicine) |
|
|
|
| References |
(1) |
Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL,
Lose EJ: Biology of the syndecans: a family of transmembrane heparan
sulfate proteoglycans. Ann. Rev. Cell Biol., 8:365-393, 1992. |
|
(2) |
Rosenberg RD, Schworak NW, Lui J, Schwartz JJ, Zhang L: Heparan
sulfate proteoglycans of the cardiovascular system. Specific structures
emerge but how is synthesis regulated ? J. Clin. Invest., 99:2062-2070,
1997. |
|
(3) |
Veugelers M, Vermeesch J, Reekmans G, Steinfeld R, Marynen P,
David G: Characterization of glypican-5 and chromosomal localization
of human GPC5, a new member of the glypican gene family. Genomics
40: 24-30, 1997. |
|
|
|
|
|
|
| Dec.15, 1997 |
|
|
|
|
|
|
|



