

 |
 |
Growth Factors and Heparan Sulfate |  | |||||||||||||
 |
To exert their various biological activities, heparan sulfate (HS) and heparin bind to specific proteins which have been identified over the years. Table 1 lists some of the HS and heparin-binding proteins, grouped according to functions they performed. Especially HS and heparin have been implicated in the regulation of cell growth and differentiation, although the detailed mechanisms underlying their actions are not yet completely understood. There is an emerging consensus that these polymers may be exerting their effects by modulating the activity of growth factors. Various growth factors such as fibroblast growth factors (FGFs), vascular endothelial growth factor, heparin-binding epidermal growth factor, and hepatocyte growth factor (HGF), etc. bind to HS and heparin and form tight complexes. The functional importance of such interactions has recently been studied. HS and heparin especially have significant potential to regulate the mitogenecity of several members of FGF family, and the interactions are prerequisites for binding of the growth factors to their high affinity transmembrane receptor and biological activities of the growth factors. FGF family of growth factors comprises at least nine members that have a broad target-cell specificity. Structural studies on HS and heparin have shown that IdoA 2-O-sulfate and GlcN N-sulfate are essential to FGF-2 binding and decasaccharide or larger can stimulate mitogenic activity. In contrast with FGF-2, a high content of the GlcNS 6-O-sulfate is required for specific interactions with FGF-1 and FGF-7. Furthermore, the binding of HS and heparin to HGF seems to involve unique domains with predominantly non-sulfated IdoAs, and the affinity is most closely associated with the content of GlcNS 6-O-sulfate. Thus, the presence of various O-sulfate groups in addition to N-sulfate groups in the domains of HS and heparin can provide specific affinity to each growth factor. | |||||||||||||
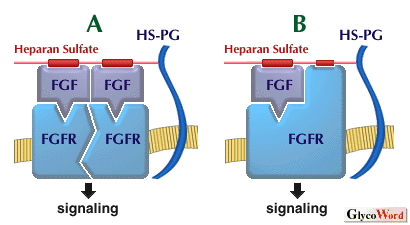 Figure Legend Models of interactions between HS-PG (heparan sulfate proteoglycan), FGF, and FGFR (FGF recepter). (A) The HS chain of HSPG interacts with two or more FGF molecules so as to induce dimerization required for receptor signaling. (B)Receptor signaling requires the formation of a trimeric complex containing HS, FGF, and FGFR. | ||||||||||||||
| How do HS and heparin facilitate the binding of FGF to FGF receptor and elicit its biological activity? Although there are various explanations for this, the two most likely possibilities are displayed in the figure. HS and heparin bind to FGFs in a multivalent manner and induce oligomerization of the FGFs (Figure-A), which is responsible for FGF receptor dimerization, activation, and signaling (Figure-A). In contrast, HS and heparin promote the activity of FGF if it is capable of simultaneously binding to regions on both the growth factor and its receptor (Figure-B). Although it has not been conclusively demonstrated which model is the correct one, further study will provide the answer. The mechanisms are also probably more complicated. Here I mainly described the interactions between HS/heparin and FGFs. However, HS and heparin in fact interact not only with FGFs but also with various other heparin-binding growth factors and modify their activities. Some concepts concerning FGFs also apply to other growth factors. | ||||||||||||||
| Masayuki Ishihara (National Defense Medical College, Research Institute) | ||||||||||||||
| ||||||||||||||
| Dec.15, 1997 | ||||||||||||||
| ||||||||||||||