| Annexin Family Proteins and Lectin Activity |  |
|
 |
Annexins comprise a family of calcium- and phospholipid-binding proteins. Over 20 members have been found in all eukaryotic kingdoms as well as plants and animals with the exception of fungi. Annexins have molecular weights ranging between 30 and 40 kDa (only annexin VI is 66 kDa) and possess striking structural features. Annexins' aminoterminal domains are diverse in sequence and length (ranging from 11 to 196) on each annexin member. In contrast the carboxyterminal regions consisting of four (eight only for annexin VI) a-helical domains composed of about 70 amino acid residues are well conserved among annexins. The calcium- and phospholipid-binding sites are located in the carboxyterminal domains.
At least one of the members can be found expressed in nearly every eukaryotic cell types. Almost all cells produce several kinds of annexins simultaniously and the expression levels are rather high. Why do they need annexins in plenty ? It may be helpful in defining the question that the task of annexins is not a unique and annexins have to interact with various ligands both in outside and inside of cells to act multiple roles.
Annexins bind to phosphatidylserine, phosphatidylethanolamine, and phosphatidylinositol, which are known to be rich in the inner leaflet of plasma membrane and hardly appear on cell surface, in contrast to phosphatidylcholin and sphingomyelin, which are major components of the outer leaflet of plasma membrane. However, the latter they are not recognized by annexins. It is reasonable to assume that the proposed functions of intracellular annexins include regulation of phospholipase A2 activity due to sequesteration of substrate phospholipids, and involvement in vesicular transport and trafficking, endocytosis, and exocytosis. Shifts in subcellular locations (from the cytosol to membrane) are observed on some intracellular annexins, suggesting the active movement of annexins corresponding to dynamic lipid vesicle transport. | |
|
 |
| Figure Legend
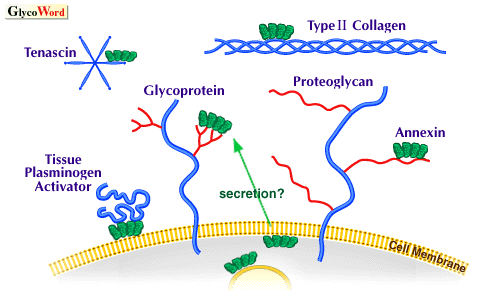
Schematic representation of proposed ligands for annexins in extracellular space.
Annexin II binds to tissue plasminogen activator and tenascin. Anchorin CII isolated as a collagen-receptor is identical with annexin V. Annexin IV, V, and VI exhibit carbohydrate-binding activity, however exact glycoconjugate ligands with physiological relevance remaind to be elucidated. | |
|
|
Furthermore annexins are exported from cytosol to the outside of cells across the plasma membrane by unknown mechanisms. Although annexins lack hydrophobic signal peptides, secretion and expression on cell surface experienced by some annexins are evident in some cell types. After being exported outside of cells, some annexins have been shown to function as receptors for extracellular proteins; annexin V binds to collagen and annexin II binds to tissue plasminogen activator and tenascin. Moreover, there exists evidence that annexins interact with glycoconjugates. First, annexin IV was identified as a lectin binding to carbohydrate moieties of sialoglycoproteins and glycosaminoglycans in the presence of calcium; afterwards it was shown that annexin V and VI and some other annexins exhibit similar lectin activity. The localization sites of annexin IV in the major expression tissues, the kidney and pancreas, and the result of in vitro binding assay of several glycoconjugates suggest that annexin IV is involved in the formation of apical sorting (secretory) vesicles due to the interaction with some GPI-anchored glycoproteins and proteoglycans. Exocrine-type neurotrophic activity is found in annexin V and involvement of some annexins in cell-adhesion (or inhibition of cell-adhesion) has been found. Future studies should aim at identifying endogenous glycoconjugate ligands recognized by annexins in a variety of cases and investigating the carbohydrate recognition mechanism. | |
|
| Kyoko Kojima (Ochanomizu University, Faculty of Sciences) | |
|
|
| References | (1) | Annexins. J. Mollenhauer et al., Cell Mol. Life Sci. 53, 506-556, 1997. |
| (2) | Characterization of carbohydrate-binding protein p33/41. -relation with annexin IV, molecular basis of the doublet forms (p33 and p41), and modulation of the carbohydrate binding activity by phospholipids. K. Kojima, K. Yamamoto, T. Irimura, T. Osawa, H. Ogawa, and I. Matsumoto. J. Biol. Chem., 271, 7679-7685, 1996. |
| (3) | Characterization of the heparin binding properties of annexin II tetramer. Kassam, G., Manro, A., Braat, C. E., Louie, P., Fitzpatrick, S. L., and Waisman,D. M. J. Biol. Chem., 272, 15093-15100, 1997. |
| | |
| |
| Dec.15, 1997 |
|
| |
|
|
|
|



