|
LPS and Toll-like Receptor
|
|
|
 |
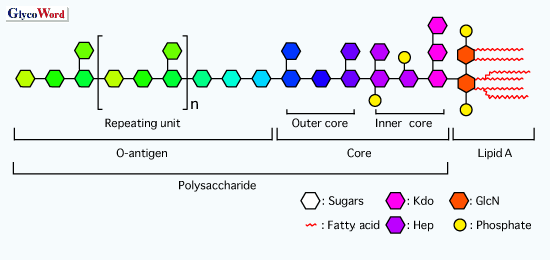
Lipopolysaccharide (LPS) is the principal outer membrane
component of Gram-negative bacteria and a strong stimulator of the innate
immunity of their host (1). LPS is an amphipathic molecule consisting
of a hydrophilic polysaccharide portion in the core region and O-antigen,
and a hydrophobic glycolipid anchor t ermed lipid A (Fig.
1). O-antigen is composed of a sequence of repeating units of oligosaccharide
specific to bacterial species and/or strains, and defines the serologic
classification of Gram-negative bacteria. The core region is an oligosaccharide
containing characteristic sugar residues, Kdo and heptose, and its chemical
variation is more limited than that of O-antigen. The core region is also
considered to possess immunogenic properties. However, the details remain
unknown. Lipid A is composed of a phosphorylated diglucosamine carrying
acyl residues, and is recognized as a primary immunostimulatory center
of LPS.
Many kinds of lipid As and their analogues have been synthesized, and
their structure-activity relationships thoroughly evaluated. The activity
of lipid A is strictly dependent on the chemical structure, e.g., chain
length and number, the positions of the acyl groups, as well as the number
of phosphate groups. Furthermore, the activity depends on the particular
mammalian species investigated. In the case of human cells, Escherichia
coli-type lipid A consisting of glucosamine disaccharide, two phosphates
and six acyl groups (Fig. 2A) showed the
strongest immuno-stimulating activity, while its counterparts possessing
different kinds or number of substituents display reduced activity. Remarkably,
the biosynthetic precursor of the lipid A having tetraacyl groups (Fig.
2B) did not show the activity and inhibited the stimulartory activity
of LPS or E. coli -type lipid A. However, both hexaacyl and tetraacyl
compounds showed similar stimulating activities in the case of murine
cells. These observations indicate that lipid A molecules are precisely
recognized by a receptor or its complex on the host cells.
CD14 was previously reported to be a LPS-binding receptor which is distributed
on the cell surface and enhances the LPS signals. But since CD14 lacks
a cytoplasmic domain, it may not be responsible directly for the signal
transduction. Recently, Toll-like receptor (TLR) family, which is a type-I
transmembrane protein expressed on immune cells such as macrophages and
dendritic cells, has been found as a major signaling receptor of the innate
immune system (2). TLRs recognize pathogen-specific molecules by their
extracellular leucine-rich repeat (LRR) domain, activate the signaling
cascade involving MyD88, IRAK, TRAF6, and NF-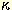 B
via its cytoplasmic Toll/IL-1 receptor (TIR) domain, and lead to the synthesis
of inflammatory mediators, like cytokines or nitric oxide. To date, more
than ten members of the TLR family have been found. Among them, TLR4 is
recognized as LPS receptors by the following facts. LPS-nonresponsive
mouse was found to carry a point mutation in the TIL domain of the TLR4
gene. TLR4 deficient (TLR4-/-) cells
were defective in their responses to LPS or lipid A. B
via its cytoplasmic Toll/IL-1 receptor (TIR) domain, and lead to the synthesis
of inflammatory mediators, like cytokines or nitric oxide. To date, more
than ten members of the TLR family have been found. Among them, TLR4 is
recognized as LPS receptors by the following facts. LPS-nonresponsive
mouse was found to carry a point mutation in the TIL domain of the TLR4
gene. TLR4 deficient (TLR4-/-) cells
were defective in their responses to LPS or lipid A.
Furthermore, MD-2, a lipid binding protein associated with the LRR domain
of TLR4, has been identified. MD-2-deficient (MD-2-/-)
cells were shown to be LPS-unresponsive, indicating the essential role
of MD-2 for the signal transduction. It was reported that MD-2 comes into
physical contact with LPS and is responsible for the species-dependent
activation by lipid A derivatives. These observations reveal that MD-2
acts as an adapter molecule of TLR4 and supports the recognition of LPS
or lipid A. Ligand recognition using an adapter molecule is characteristic
only of TLR4. However, direct evidence of binding between TLR4, MD-2 and
LPS or lipid A has not yet been reported. Much remains to be elucidated
concerning the molecular mechanisms of LPS and lipid A recognition. |
|
|
 |
| |
| Fig. 1
Schematic structure of LPS. |
| |
| |
|
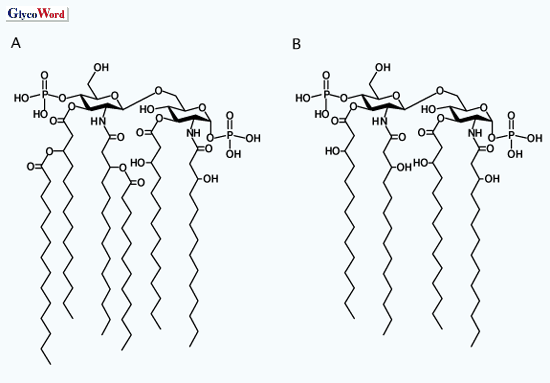 |
| |
| Fig. 2 Chemical structure of lipid A. |
| |
| |
|
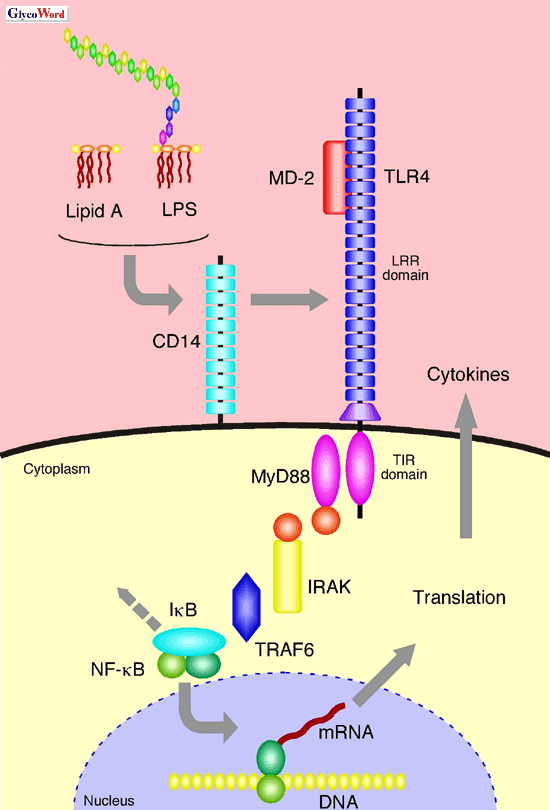 |
| |
| Fig. 3 Recognition and signaling pathway of LPS and lipid A. |
|
|
|
Masahito Hashimoto (Asahi University School of Dentistry) |
|
|
|
|
|
| References |
(1) |
Ulmer, A.J., Rietschel, E.T., Z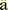 hringer,
U., Hein, H. Lipopolysaccharide: structure, bioactivity, receptors,
and signal transduction. Trends Glycosci. Glycotechnol., 14, 53-68,
2002; Alexander, C., Z hringer,
U., Hein, H. Lipopolysaccharide: structure, bioactivity, receptors,
and signal transduction. Trends Glycosci. Glycotechnol., 14, 53-68,
2002; Alexander, C., Z hringer,
U. Chemical structure of lipid A - the primary immunomodulatory
center of bacterial lipopolysaccharides. ibid, 69-86; Takada, H.,
Kotani, S. Structural requirements of lipid A for endotoxicity and
other biological activities. Crit. Rev. Microbiol., 16, 477-523,
1989. hringer,
U. Chemical structure of lipid A - the primary immunomodulatory
center of bacterial lipopolysaccharides. ibid, 69-86; Takada, H.,
Kotani, S. Structural requirements of lipid A for endotoxicity and
other biological activities. Crit. Rev. Microbiol., 16, 477-523,
1989. |
|
(2) |
Akira, S., Takeda, K., Kaisho, T. Toll-like receptors: critical
proteins linking innate and acquired immunity. Nat. Immunol., 2,
675-680, 2001. |
|
(3) |
Miyake, K. Innate recognition of lipopolysaccharide by CD14 and
toll-like receptor 4-MD-2: unique roles for MD-2. Int. Immunopharmacol.,
3, 119-128, 2003. |
|
|
|
|
|
| Nov. 12, 2003 |
|
|
|
|
|
|
|



