| Carbohydrate Antigens in Development and Differentiation |  |
|
 |
Glycoconjugates are major components of the outer surface of animal cells, and their carbohydrate structures change dramatically during development. Specific sets of carbohydrates are characteristic of different stages of differentiation and very often these carbohydrates are recognized by specific antibodies, thus providing differentiation antigens. In the mature organism, expression of distinct carbohydrates is eventually restricted to specific cell types. Aberrant expression of cell surface carbohydrates is very often associated with malignant transformation. They usually represent those present in early development and differentiation in normal cells, thus we call them oncodevelopmental or oncodifferentiation carbohydrates (or antigens).
Carbohydrate differentiation antigens can serve as ligands for carbohydrate-binding proteins.
One of these antigens is the Lex antigen (or SSEA-1, stage specific embryonic antigen-1) originally discovered in the early embryo of the mouse. This carbohydrate appears to be involved in compaction in early embryogenesis but its presence is restricted to very few tissues and organs in adult. The sialylated form of Lex, sialyl Lex, having a NeuNAc alpha2-3Gal beta1-4(Fuc alpha1-3)GlcNAc-R structure, is a typical differentiation antigen present in leukocytes. There is direct evidence that sialyl Lex present on leukocytes is recognized by E- and P-selectin present in platelets and endothelial cells. Interestingly, the sulfated form of sialyl Lex is present on a specialized endothelium (high endothelial venules) and serves as a trafficking signal for lymphocytes to enter lymphoid circulation from blood circulation. These results indicate that carbohydrate-protein interaction takes place through two-way communication in this system. The expression of sialyl Lex and sulfated Lex is thus restricted to specific cell types. This specificity is achieved by specific expression of fucosyltransferase VII and possibly a sulfotransferase in leukocytes and high endotherial venules.
The expression of developmental carbohydrate antigen is also regulated by the expression levels of underlying carbohydrates.
In particular, the synthesis of a branch provides various possibilities for those oligosaccharides. Selectins preferentially bind to sialyl Lex and sulfated sialyl Lex present in mucin-type O-glycans, when core 2 branched structures are present (Fig. A). The expression of core 2 O-glycans is highly regulated during development. In T cell development, immature thymocytes in cortical thymus express core 2 branched O-glycans but the synthesis of core 2 O-glycans is turned off in relatively mature medullary thymocytes. T cells in the peripheral blood do not express core 2 O-glycans, but after activation, T cells acquire core 2 O-glycans. Moreover, T cells in peripheral blood synthesize core 2 O-glycans under pathological conditions such as Wiskott-Aldrich syndrome, AIDS and leukemia. These core 2 branched O-glycans apparently reduce T cell and B cell immune response, which is likely a cause of immunodeficiency in these diseases. The expression of core 2 O-glycans is regulated by the transcriptional levels of core 2 beta-1,6-N-acetylglucosaminyltransferase in all of these cases.
Core 2 O-glycan synthesis also leads to the synthesis of poly-N-acetyllactosamine, which contains repeats of (Gal beta1-4GlcNAc beta1-3)n, in O-glycans (Fig. B). The expression of poly-N-acetyllactosamine allows these carbohydrates to be further modified to express differentiation antigens. Sialyl poly Lex is one of these structures and this oligosaccharide is synthesized through the concerted actions of fucosyltransferase VII and IV, uniquely present in leukocytes.
In N-glycans, the synthesis of poly-N-acetyllactosamine is increased substantially once the GlcNAc beta1-6Man alpha1-6Man beta1-4 branch is synthesized by N-acetylglucosaminyltransferase V, GlcNAc-TV. N-Glycans containing this branch usually contains tri- and tetra-antennae. The increase of tri- and tetra-antennary N-glycans is associated with malignant transformation and other biological processes such as T cell activation. These results indicate that the increase in antennae can lead to increase of poly-N-acetyllactosamine.
The increase of N-acetyllactosamine is also possible by having N-acetyllactosamine branching on linear poly-N-acetyllactosamine. These linear and branched N-acetyllactosamines are also developmental antigens, called i and I antigens, respectively. In human erythrocyte cells, the conversion of i to I antigen takes place after birth. The I antigen can be further modified to express other differentiation antigens such as sialyl Lex. Those sialyl Lex present in both neighboring branches of poly-N-acetyllactosamines provide a better avidity for carbohydrate-binding proteins such as antibodies and selectin. By having linear poylactosamine in fetal erythrocytes, it is thought that autoimmune response to incompatible carbohydrate antigen can be minimized.
In summary, the expression of differentiation carbohydrate antigens can be controlled by the formation of underlying branch structures and then by specific modifications to form those unique carbohydrate antigens present in non-reducing termini.
| |
|
| The biosynthetic pathways of O-glycans A and poly-N-acetyllactosaminyl O-glycans B. |
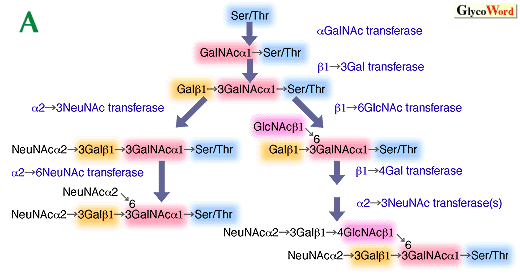
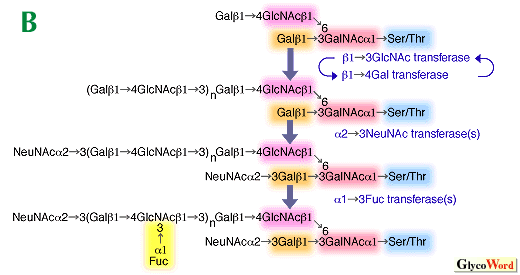 |
Figure Legend
A. Resting T cells synthesize the tetrasaccharide (bottom left) by sequential action of alpha-2,3-sialyltransferase followed by alpha-2,6-sialyltransferase. When core 2 beta-1,6-N-acetylglucosaminyltransferase is present, the branched hexasaccharide is formed (bottom right).
B. Poly-N-acetyllactosaminyl side chains can be extended from the GlcNAc beta 1-6 structure formed in the core 2 branch, when beta-1,3-N-acetylglucosaminyltransferase (i enzyme) is present. Poly-N-acetyllactosamine extensions can be further modified by alpha-1,3-fucosyltransferase forming sialyl Lex terminus. |
|
|
|
Carbohydrate differentiation antigens play a role in modulating the functions of proteins.
One of these carbohydrates is polysialic acid. Polysialic acid, a linear polymer of alpha-2,8-linked sialic acid, displays a dramatic developmental change in neuronal cell development. In fetal brain, this unique carbohydrate is attached to the neural cell adhesion molecule, N-CAM. The majority o N-CAM in adult brain, however, does not express polysialic acid but polysialylated N-CAM persists in part of the brain where neural regeneration continues in adult such as hippocampus and olfactory bulb. Polysialic acid is thought to attenuate the adhesive property of N-CAM, thereby allowing neural cells to migrate and extend axons. In various tumors, including small cell lung carcinoma and Wilm's tumor in kidney, polysialic acid is highly expressed, although normal tissues express only small amounts of polysialic acid in these organs. Polysialic acid in these tumors probably provides a tool for tumor cells to invade and migrate in metastasis. Recently, the cDNAs encoding two polysialyltransferases, PST and STX, have been cloned. PST and STX belong to a sialyltransferase gene family containing sialyl L and sialyl S motif shared by all these sialyltransferases. When PST or STX was expressed in HeLa cells synthesizing N-CAM, those HeLa substratum cells contain polysialic acid in N-CAM and facilitate neurite outgrowth better than HeLa cells expressing N-CAM alone, supporting the above hypothesis that polysialic acid plays a role in neural cell migration.
The expression profile of PST and STX during mouse development demonstrated that polysialic acid synthesis is primarily regulated by the transcription levels of these two enzymes. After birth, STX expression declines substantially while the expression of PST persists in adult brain. Polysialic acid synthesis is also controlled by a change in concentration of Ca2+, which is an activator for PST and STX. By reducing the concentrations of intracellular Ca2+, polysialic acid synthesis is reduced even when the levels of PST or STX transcripts are barely reduced.
This exemplifies the regulation of carbohydrate differentiation antigen by another mechanism and the amount of polysialic acid is regulated by the levels of the transcription for the polysialyltransferases and the concentration of activator(s), Ca2+.
| |
|
| Minoru Fukuda (The Burnham Institute, La Jolla, CA) | |
|
|
| References | (1) | Fukuda M : Possible roles of tumor-associated carbohydrate antigens. IN: Perspectives in Cancer Research, Cancer Res. 56, 2237-2244, 1996 |
| (2) | Tsuboi S, Fukuda M : Branched O-linked oligosaccharides ectopically expressed in transgenic mice reduce primary T-cell immune responses. EMBO J. 16, 6364-6373, 1997 |
| (3) | Brusés JL, Rutishauser U : Regulation of neural cell adhesion molecule polysialylation: Evidence for nontranscriptional control and sensitivity to an intracellular pool of calcium. J. Cell Biol. 140, 1177- 1186, 1998 |
| (4)) | Ohyama C, Tsuboi S, Fukuda M : Dual roles of sialyl Lewis X oligosaccharides in tumor metastasis and rejection by natural killer cells. EMBO J. 18, 1516-1525 1999
|
| |
|
| Jun.15, 1998 |
|
| |
|
|
|
|



