Structure and biosynthesis of O-mannosyl glycans in mammals | |||||||||||||||||||||
 |
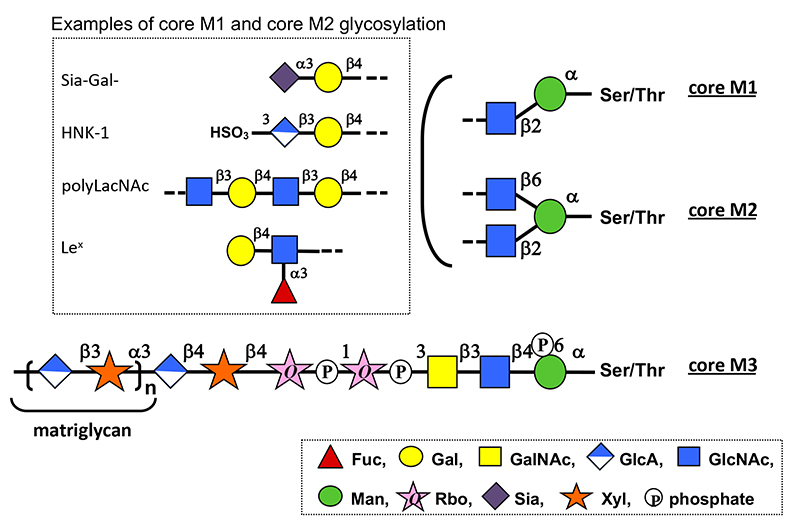
O-Mannosyl glycans are a type of O-glycan in which the reducing terminal mannose (Man) is attached to a protein via serine (Ser) or threonine (Thr) residues (1). Various structures of O-mannosyl glycans identified can be classified into three types: core M1, N-acetylglucosamine (GlcNAc)β1-2Man; core M2, GlcNAcβ1-2(GlcNAcβ1-6)Man; and core M3, N-acetylgalactosamine (GalNAc)β1-3GlcNAcβ1-4Man. (Fig. 1) The non-reducing ends of core M1 and core M2 glycans are modified with various structures found in other O- and N-glycans. In contrast, core M3 glycan has a unique structure containing two ribitol-5-phosphates (Rbo5P) linked via phosphodiester bonds and a long-chain repeating structure consisting of glucuronic acid (GlcA) and xylose (Xyl) disaccharide units called “matriglycan.” Core M3 glycans are found on α-dystroglycan, a cell surface receptor for extracellular matrix molecules such as laminin. Matriglycan is required for laminin binding to α-dystroglycan and contributes to formation of dystrophin–glycoprotein complex involved in skeletal muscle maintenance and brain development. Defects in the enzymes involved in core M3 glycan biosynthesis are the primary cause of α-dystroglycanopathy, a group of congenital muscular dystrophies coincident with brain malformations. α-Dystroglycanopathy is caused by the failure of α-dystroglycan to bind to laminin owing to a defect in its matriglycan. (see “Pathogenesis and therapeutic strategy of glycosylation-defective muscular dystrophy (dystroglycanopathy)”)
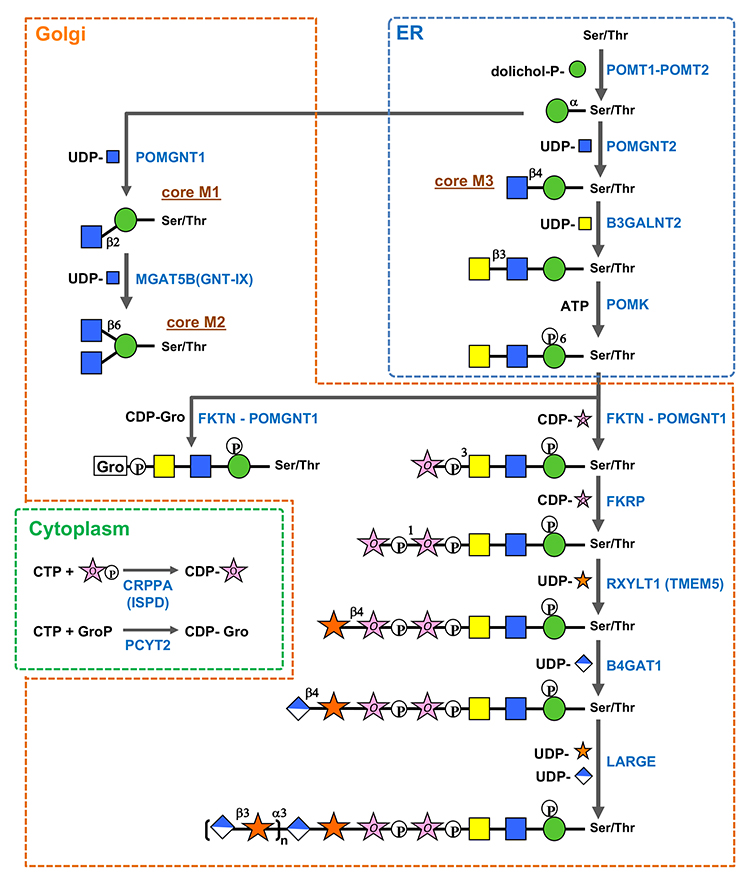
O-Mannosyl glycan biosynthesis (Fig.2) (1–8) is initiated by O-mannosyl transferases (heterocomplexes of POMT1 and POMT2) that transfer Man from dolichol phosphate mannose (Dol-P-Man) to Ser/Thr in the endoplasmic reticulum (ER). After O-Man modification, POMGNT2 functions as a key enzyme in determining whether core M1, M2, or M3 glycan biosynthesis should proceed in the ER. O-Man residues, to which GlcNAc is transferred by POMGNT2 via a β1-4 linkage, become a foundation of core M3 glycan biosynthesis. In contrast, O-Man residues not modified by POMGNT2 in the ER become modified by POMGNT1 after transport to the Golgi apparatus to form GlcNAcβ1-2Man and drive core M1/M2 glycan biosynthesis. Because MGAT5B (GNT-IX) is expressed in the brain, GlcNAc can be transferred to the C6 position of Man in core M1, and the core M2 structure is also formed. Biosynthesis of the non-reducing side structures of core M1/M2 is thought to be similar to that of other O- and N-glycans. Thr residues at positions 317, 319, and 379 of α-dystroglycan serve as potential sites where POMGNT2 forms GlcNAcβ1-4Man to modify core M3 glycans. Following the formation of GlcNAcβ1-4Man, GalNAc is transferred by B3GALNT2 to the C3 position of GlcNAc via β-linkage, and the C6 position of Man is phosphorylated by POMK. The phosphate group of Man is required for FKTN and FKRP to transfer of two Rbo5Ps in the next step (1, 2). Phosphorylated core M3 glycans by POMK translocate from the ER to the Golgi apparatus. FKTN transfers the first Rbo5P to the C3 position of GalNAc via a phosphodiester linkage. Rbo5P transfer by FKTN in vivo requires interaction with POMGNT1. POMGNT1 possesses a catalytic and a lectin domain. The catalytic domain is involved in core M1 formation as a β1-2GlcNAc transferase, whereas the lectin domain contributes to FKTN recruitment to core M3 by binding to GalNAcβ1-3GlcNAc in core M3. FKRP then transfers the second Rbo5P to the C1 position of the first Rbo5P via a phosphodiester linkage. CDP-ribitol (CDP-Rbo) is the donor substrate for Rbo5P transfer. CDP-Rbo is produced from CTP and Rbo5P via CRPPA/ISPD. The glycerol-3-phosphate (Gro3P) modification instead of the Rbo5P modification has been identified in core M3 glycan on α-dystroglycan (3). Similar to Rbo5P, FKTN and FKRP can transfer Gro3P to core M3 glycan using CDP-glycerol (CDP-Gro) as a donor substrate (4). However, GroP-modified core M3 produced by FKTN cannot serve as an acceptor substrate for FKRP. Kinetic analysis results indicate that CDP-Gro competitively inhibits the Rbo5P transferase activities of both FKTN and FKRP. These results suggest that CDP-Gro inhibits matriglycan formation. CDP-Gro is produced from CTP and Gro3P by PCYT2 (5,6). After tandem Rbo5P modification, RXYLT1 transfers Xyl to the second Rbo5P via a β1-4 linkage, and then B4GAT1 transfers GlcA to Xyl via a β1-4 linkage. Finally, LARGE, which has two catalytic domains responsible for α1,3-xylosyltransferase and β1,3-glucuronosyltransferase, alternately and sequentially transfers Xyl and GlcA, respevtively, to form the (-3GlcAβ1-3Xylα1) repeat of matriglycan. The structure of the first GlcA-Xyl unit linked to Rbo5P, GlcAβ1-4Xylβ1, differs from that of 3GlcAβ1-3Xylα1 of matriglycan. Since β1,3-glucuronosyltransferase or α1,3-xylosyltransferase of LARGE is unable to transfer Xyl to Rbo5P or GlcA to the β-linked Xyl, the GlcAβ1-4Xylβ1 structure formed by RXYLT1 and B4GAT1 is required as a linker for matriglycan elongation. In addition, the sulfation of GlcA on core M3 glycan inhibits matriglycan elongation (7,8). Advanced future analyses will help identify more proteins modified with O-mannosyl glycans and reveal the functions of various O-mannosyl glycan structures and regulatory mechanisms of O-mannosyl glycan biosynthesis.
Hiroshi Manya
| ||||||||||||||||||||
| References | |
|---|---|
| (1) | Manya H, Endo T: Glycosylation with ribitol-phosphate in mammals: New insights into the O-mannosyl glycan. Biochim. Biophys. Acta. 1861, 2462-2472, 2017 |
| (2) | Kuwabara N, Imae R, Manya H, Tanaka T, Mizuno M, Tsumoto H, Kanagawa M, Kobayashi K, Toda T, Senda T, Endo T, Kato R: Crystal structures of fukutin-related protein (FKRP), a ribitol-phosphate transferase related to muscular dystrophy. Nat. Commun. 11, 303, 2020 |
| (3) | Yagi H, Kuo CW, Obayashi T, Ninagawa S, Khoo KH, Kato K: Direct mapping of additional modifications on phosphorylated O-glycans of α-dystroglycan by mass spectrometry analysis in conjunction with knocking out of causative genes for dystroglycanopathy. Mol. Cell. Proteomics 15, 3424-3434, 2016 |
| (4) | Imae R, Manya H, Tsumoto H, Osumi K, Tanaka T, Mizuno M, Kanagawa M, Kobayashi K, Toda T, Endo T: CDP-glycerol inhibits the synthesis of the functional O-mannosyl glycan of α-dystroglycan. J. Biol. Chem. 293, 12186-12198, 2018 |
| (5) | Imae R, Manya H, Tsumoto H, Miura Y, Endo T: PCYT2 synthesizes CDP-glycerol in mammals and reduced PCYT2 enhances the expression of functionally glycosylated α-dystroglycan. J. Biochem. 170, 183-194, 2021 |
| (6) | Umezawa F, Natsume M, Fukusada S, Nakajima K, Yamasaki F, Kawashima H, Kuo C-W, Khoo K-H, Shimura T, Yagi H, Kato K: Cancer malignancy is correlated with upregulation of PCYT2-mediated glycerol phosphate modification of α-dystroglycan. Int. J. Mol. Sci. 23, 6662, 2022 |
| (7) | Nakagawa N, Manya H, Toda T, Endo T, Oka S: Human natural killer-1 sulfotransferase (HNK-1ST)-induced sulfate transfer regulates laminin-binding glycans on α-dystroglycan. J. Biol. Chem. 287, 30823-32, 2012 |
| (8) | Sheikh MO, Venzke D, Anderson ME, Yoshida-Moriguchi T, Glushka JN, Nairn AV, Galizzi M, Moremen KW, Campbell KP, Wells L: HNK-1 sulfotransferase modulates α-dystroglycan glycosylation by 3-O-sulfation of glucuronic acid on matriglycan. Glycobiology 30, 817-829, 2020 |
Mar. 15, 2024