| |
In 1970, Berson and Yalow defined insulin resistance as
"Decreased ability of cells or tissues to respond to physiological
levels of insulin.” In the developed countries, pathological significance
of insulin resistance is highly recognized as the cause of lifecycle -
related diseases including hyperlipidemia, hypertension, hyperinsulinemia,
and more significantly, obesity and type 2 diabetes. In the state of insulin
resistance, the metabolic signals of insulin in liver, muscle and adipose
tissues were selectively impaired. In fact, knockout mice lacking IRS-1
exhibited insulin resistance with hyperinsulinemia 1).
Adipose tissues from various species, including human, rat and mouse,
contain GM3 as the most abundant type of ganglioside. It is known that,
when GM3 is added exogenously to cultured cells expressing EGF receptor
2) and IR
3), the inhibition of ligand-stimulated receptor
autophosphorylation is observed. Moreover, there is a possiblility that
endogenous GM3 may indeed regulate insulin signaling since IR exists in
the membrane microdomains 4),
the restricted membrane micro-compartment concentrating sphingomyelin,
cholesterol and glycosphingolipids including GM3. We present evidence
for the first time that the acquisition of a state of insulin resistance
in adipocytes induced by TNF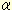 may depend upon increased GM3 biosynthesis
through upregulation of GM3 synthase gene expression and the GM3 may function
as an inhibitor in insulin signaling during the chronic exposure of adipocytes
to TNF may depend upon increased GM3 biosynthesis
through upregulation of GM3 synthase gene expression and the GM3 may function
as an inhibitor in insulin signaling during the chronic exposure of adipocytes
to TNF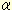 5).
Here I describe our recent observation demonstrating that GM3 suppressed
insulin metabolic signaling by affecting the functions of microdomains. 5).
Here I describe our recent observation demonstrating that GM3 suppressed
insulin metabolic signaling by affecting the functions of microdomains.
Insulin resistance in type 2 diabetes and TNF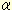
Genetically obese db/db mice, ob/ob mice and Zucker
rats exhibit insulin resistance, and TNF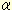 mRNA was expressed in their adipocytes at high levels compared to the
control (lean) animals as well as obese diabetic human subjects. When
the soluble TNF receptor-IgG kimeric protein was injected into Zucker
rats to absorb TNF
mRNA was expressed in their adipocytes at high levels compared to the
control (lean) animals as well as obese diabetic human subjects. When
the soluble TNF receptor-IgG kimeric protein was injected into Zucker
rats to absorb TNF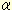 ,
the state of insulin resistance was ameliorated. In addition, ob/ob mice
lacking TNF ,
the state of insulin resistance was ameliorated. In addition, ob/ob mice
lacking TNF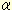 gene did not develop. From these observation, the considerable attention
has been focused in TNF
gene did not develop. From these observation, the considerable attention
has been focused in TNF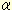 over production by adipocytes accumulated neutral fats as the cause of
insulin resistance.
over production by adipocytes accumulated neutral fats as the cause of
insulin resistance.
Ganglioside GM3 as inducer of insulin resistance 5)
When mouse 3T3-L1 adipocytes were cultured in low concentrations of TNF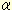 which do not cause the generalized suppression of adipocyte gene expression
including IRS-1 and GLUT-4, interference to insulin action by TNF
which do not cause the generalized suppression of adipocyte gene expression
including IRS-1 and GLUT-4, interference to insulin action by TNF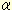 occurred. This requires prolonged treatment (at least 72 h), unlike many
acute effects of this cytokine. The slowness of the effect suggests that
TNF
occurred. This requires prolonged treatment (at least 72 h), unlike many
acute effects of this cytokine. The slowness of the effect suggests that
TNF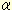 induces
the synthesis of an inhibitor that is the actual effector. We demonstrated
that the state of insulin resistance in adipocytes treated with 0.1 nM
TNF induces
the synthesis of an inhibitor that is the actual effector. We demonstrated
that the state of insulin resistance in adipocytes treated with 0.1 nM
TNF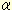 was accompanied
by a progressive increase in cell surface GM3. This was mirrored with
increases in cellular GM3 content, GM3 synthase activity and GM3 synthase
mRNA content, indicating that TNF was accompanied
by a progressive increase in cell surface GM3. This was mirrored with
increases in cellular GM3 content, GM3 synthase activity and GM3 synthase
mRNA content, indicating that TNF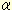 upregulates GM3 synthesis at the transcriptional level in cultured adipocytes.
We were able to extend these in vitro observations to intact animals,
since GM3 synthase mRNA contents in adipose tissues from the obese Zucker
fa/fa rats and ob/ob mice were significantly high in comparison
to the lean animals 5).
We are currently analyzing the level of GM3 expression in adipose tissues
from other rodent models as well as from humans with type2 diabetes.
upregulates GM3 synthesis at the transcriptional level in cultured adipocytes.
We were able to extend these in vitro observations to intact animals,
since GM3 synthase mRNA contents in adipose tissues from the obese Zucker
fa/fa rats and ob/ob mice were significantly high in comparison
to the lean animals 5).
We are currently analyzing the level of GM3 expression in adipose tissues
from other rodent models as well as from humans with type2 diabetes.
To elucidate whether the increased GM3 in 3T3-L1 adipocytes treated with
TNF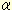 is involved
in insulin resistance, we used an inhibitor of glucosylceramide synthase,
D-PDMP (See Glycoword GL-46), to deplete cellular glycosphingolipids derived
from glucosylceramide. D-PDMP proved able to counteract TNF-induced increase
of GM3 content in adipocytes and completely normalize the TNF-induced
defect in tyrosine phosphorylation of IRS-1 in response to insulin stimulation
5). These findings
are supported by the recent unpublished observation that knockout mouse
lacking GM3 synthase exhibits enhancement of insulin signaling6). is involved
in insulin resistance, we used an inhibitor of glucosylceramide synthase,
D-PDMP (See Glycoword GL-46), to deplete cellular glycosphingolipids derived
from glucosylceramide. D-PDMP proved able to counteract TNF-induced increase
of GM3 content in adipocytes and completely normalize the TNF-induced
defect in tyrosine phosphorylation of IRS-1 in response to insulin stimulation
5). These findings
are supported by the recent unpublished observation that knockout mouse
lacking GM3 synthase exhibits enhancement of insulin signaling6).
Regulatotry roles of microdomains in insulin signaling
Insulin-mediated internalization of IR into endosomes from plasma membranes
and the intracellular movement of IRS-I to endosomes were significantly
suppressed in 3T3-L1 adipocytes treated with TNF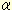 ,
leading to the inhibition of IR-IRS-1 signalosome formation. Moreover,
in the adipocytes-acquired insulin resistance by TNF ,
leading to the inhibition of IR-IRS-1 signalosome formation. Moreover,
in the adipocytes-acquired insulin resistance by TNF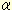 ,
IR accumulated less in the detergent-insobule low density membrane fractions
(microdomains) and shifted to the high density fractions. Interestingly,
under the state of insulin resistance, the IR – IRS-I signaling
was selectively impaired without affecting the insulin-mediated MAPK activations.
These observation strongly suggest that the defect of insulin’s
metabolic signal in insulin resistance may be attributed to the loss of
IR from microdomains and impaired endocytosis or IR(Fig.
2 and 3). ,
IR accumulated less in the detergent-insobule low density membrane fractions
(microdomains) and shifted to the high density fractions. Interestingly,
under the state of insulin resistance, the IR – IRS-I signaling
was selectively impaired without affecting the insulin-mediated MAPK activations.
These observation strongly suggest that the defect of insulin’s
metabolic signal in insulin resistance may be attributed to the loss of
IR from microdomains and impaired endocytosis or IR(Fig.
2 and 3).
Further work is in progress in our laboratory to pursue the functional
and structural changes of microdomains in the state of insulin resistance
and type 2 diabetes to elucidate the fundamental roles of GM3 employing
bioinformatics including proteome, metabolome and structural biology.
|
|
|
| References |
(1) |
Kadowaki, T. J. Clin. Invest. 106, 459-465 (2000) |
|
(2) |
Bremer, E. G., Schlessinger, J., and Hakomori, S. J.Biol.Chem.
261, 2434-2440 (1986) |
|
(3) |
Nojiri, H., Stroud, M., and Hakomori, S. J. Biol. Chem.
266, 4531-4537 (1991) |
|
(4) |
Gustavsson, J., Parpal, S., Karlsson, M., Ramsing, C., Thorn,
H., Borg, M., Lindroth, M., Peterson, K.J., Magnusson, K, and Stralfors,
P. FASEB J. 13, 1961-1971 (1999) |
|
(5) |
Tagami, S., Inokuchi, J., Kabayama, K., Yoshimura, H., Kitamura,
F,. Uemura, S., Ogawa, C., Ishii, A., Saito, M., Ohtsuka, Y., Sakaue,
S., and Igarashi, Y.
J. Biol. Chem. 277, 3085-2216 (2002) |
|
(6) |
Yamashita, T., Hashiramoto, A., Haluzik, M., Mizukami, H., Beck,
S., Norton, A., Kono, M., Tsuji, S., Daniotti, J.L., Werth, N.,
Sandhoff, R., Sandhoff, K., and Proia, R.L. Proc. Natl. Acad,
Sci. USA, 100, 3445-3449(2003) |
|
|
|
|
|



