 |
Gangliosides, sialic acid-containing glycosphingolipids,
are present in surface membranes of cells and are thought to play important
functional roles in regulating a wide range of biological processes, including
cell surface interactions, cell differentiation and transmembrane signaling.
Human plasma membrane-associated sialidase (NEU3) is a unique enzyme hydrolyzing
gangliosides specifically1,2),
and therefore has been suggested to participate in cell surface events
through modulation of gangliosides. Here we demonstrate that mice overexpressing
NEU3 develop diabetic phenotype by 18-22 weeks associated with hyperinsulinemia,
islet hyperplasia and increased 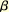 -cell
mass3). Compared
to the wild type, insulin-stimulated phosphorylation of the insulin receptor
and insulin receptor substrate I was significantly reduced, leading to
retardation in post receptor insulin signaling. In response to insulin,
NEU3 was found to undergo tyrosine-phosphorylation and associate with
Grb-2 protein, thus being activated and causing negative regulation of
insulin signaling. In fact, accumulation of GM1 and GM2, the possible
sialidase products in transgenic tissues, caused inhibition of IR phosphorylation
in vitro, and blocking of association with Grb-2 resulted in reversion
of impaired insulin signaling. The data indicate that NEU3 is a new molecule
in insulin signaling, and the mice can serve as a valuable model for human
diabetes mellitus. -cell
mass3). Compared
to the wild type, insulin-stimulated phosphorylation of the insulin receptor
and insulin receptor substrate I was significantly reduced, leading to
retardation in post receptor insulin signaling. In response to insulin,
NEU3 was found to undergo tyrosine-phosphorylation and associate with
Grb-2 protein, thus being activated and causing negative regulation of
insulin signaling. In fact, accumulation of GM1 and GM2, the possible
sialidase products in transgenic tissues, caused inhibition of IR phosphorylation
in vitro, and blocking of association with Grb-2 resulted in reversion
of impaired insulin signaling. The data indicate that NEU3 is a new molecule
in insulin signaling, and the mice can serve as a valuable model for human
diabetes mellitus. |
|
|
| References |
(1) |
Wada, T., Yoshikawa, Y., Tokuyama, S., Kuwabara, M., Akita, H.,
and Miyagi, T.: Cloning, expression, and chromosomal mapping of
a human ganglioside sialidase. Biochem. Biophys. Res. Commun.
261, 21-27 (1999). |
|
(2) |
Wang, Y., Yamaguchi, K., Wada, T., Hata, K., Zhao, X., Fujimoto,
T., and Miyagi, T.: A close association of the ganglioside-specific
sialidase Neu3 with caveolin in membrane microdomains. J. Biol.
Chem. 277, 26252-26259 (2002) |
|
(3) |
Sasaki, A., Hata, K., Suzuki, S., Sawada, M., Wada, T., Yamaguchi,
K., Obinata, M., Tateno, H., Suzuki, H., and Miyagi, T.: Overexpression
of plasma membrane-associated sialidase attenuates insulin signaling
in transgenic mice. J. Biol. Chem. 278,
27896-27902 (2003). |
|
|
|
|
|



