|
Recent Progress in Technologies and Applications of Glycogene-manipulated Mice
|
 |
|
 |
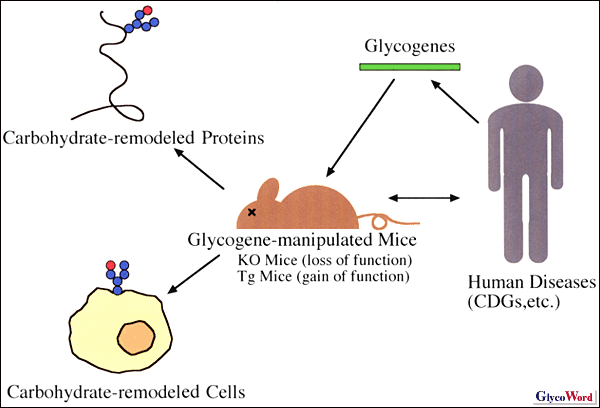
One of powerful method for the study of pleiotropic functions of carbohydrates in a body is to generate gene-manipulated mice of glycogenes encoding glycosyltransferases, glycosidases and nucleotide sugar transporters which are involved in carbohydrate biosynthesis. There are two types of gene-manipulated mice. Transgenic (Tg) mice are gain-of-function type mutations, which are generated by microinjecting exogenous DNA into pronuclei of fertilized eggs. Gene-knock-out (KO) mice are loss-of-function type mutations, which are generated using embryonic stem (ES) cells in which a targeted gene is disrupted by homologous recombination. Recent technological progress such as the Cre-loxP system and the tetracycline ON/OFF system enables us to manipulate a targeted gene on our own way to generate conditional and inducible KO mice, in which the targeted gene is disrupted in a tissue-specific and inducible manner, respectively.
The first application of the KO technology to glycogenes was the generation of N-acetylglucosaminyltransferase-I (GnT-I) KO mice in 1994. GnT-I KO mice were embryonic lethal due to the loss of complex and hybrid N-glycan biosynthesis because GnT-I undertakes the first step of complex and hybrid N-glycosylation (1, 2). The papers describing GnT-I KO mice clarify the indispensable role of carbohydrates for living animals. After that, according to successive cloning of glycogenes, Tg mice and KO mice of these glycogenes have been successively generated. Many glycogenes, however, are found to form a gene family and the disruption of one of gene family members does not cause any effect on the mouse. Detailed explanations of individual glycogens can be found in a recent review (3). Recent topics concerning glycogene-manipulated mice are discussed below. |
|
|
 |
|
|
|
|
KO mice deficient in an  -mannosidase-II ( -mannosidase-II ( -Man-II) gene which works after GnT-I in the biosynthesis of N-glycans developed dyserythropoietic anemia due to loss of the erythrocyte complex N-glycans (4). The phenotype of -Man-II) gene which works after GnT-I in the biosynthesis of N-glycans developed dyserythropoietic anemia due to loss of the erythrocyte complex N-glycans (4). The phenotype of  -Man-II KO mice correlates with human congenital dyserythropoietic anemia type II (CDA-II), suggesting that -Man-II KO mice correlates with human congenital dyserythropoietic anemia type II (CDA-II), suggesting that  -Man-II is a causal gene of the syndrome. -Man-II is a causal gene of the syndrome.
It is known that highly branched N-glycans are correlated with tumor malignancies. Tumor growth and metastasis induced by the polyomavirus middle T oncogene were suppressed in KO mice deficient in  -1,6-N-acetylglucosaminyltransferase-V (GnT-V) which is involved in the biosynthesis of branched N-glycans (5). This paper clearly demonstrates the important role of carbohydrates in tumorigenesis. -1,6-N-acetylglucosaminyltransferase-V (GnT-V) which is involved in the biosynthesis of branched N-glycans (5). This paper clearly demonstrates the important role of carbohydrates in tumorigenesis.
Much information on glycosyltransferases participating in the biosynthesis of selectin ligands has been collected. Inflammatory responses and lymphocyte homing were impaired in KO mice deficient in fucosyltransferase-VII (FucT-VII), which is involved in fucosylation of sialyl Lewis x, a major selectin ligand, while only inflammatory responses were suppressed in core 2  -1,6-N-acetylglucosaminyltransferase (C2 GlcNAc-T) KO mice, because both types of mice were impaired in selectin ligand biosynthesis (6,7). Although human mutation in these glycosyltransferase genes has not been identified yet, patients with leukocyte adhesion deficiency type II (LAD II) (a.k.a. CDG-IIc) showing similar phenotypes of these KO mice are reported to have a mutation in the GDP-fucose transporter gene. -1,6-N-acetylglucosaminyltransferase (C2 GlcNAc-T) KO mice, because both types of mice were impaired in selectin ligand biosynthesis (6,7). Although human mutation in these glycosyltransferase genes has not been identified yet, patients with leukocyte adhesion deficiency type II (LAD II) (a.k.a. CDG-IIc) showing similar phenotypes of these KO mice are reported to have a mutation in the GDP-fucose transporter gene.
Several KO mice deficient in glycogenes involved in proteoglycan biosynthesis have been generated. Surprisingly, mutant mice deficient in heparan sulfate 2-O-sulfotransferase (HS2ST) generated by gene trap mutation were embryonic lethal due to renal agenesis (8). It is suggested that blockage of signaling from growth factors such as FGF may be a cause of kidney development failure because heparan sulfate is required for interactions between FGF and its receptor.
As shown above, KO mice deficient in glycogenes represent very interesting phenotypes in higher biological phenomena such as development, immune system, neural system and oncology to elucidate biological significance of carbohydrates in a body. However, molecular mechanisms of how carbohydrates exert their biological functions remain to be elucidated. A variety of abnormalities in many tissues caused by null mutation of glycogenes in a whole body make it difficult to analyze carbohydrate functions. It is important to generate conditional KO mice to analyze the functions of glycogenes in individual tissues in the future. |
|
|
Masahide Asano
(Institute for Experimental Animals, Graduate School of Medical Science,
Kanazawa University) |
|
|
|
|
|
| References |
(1) |
Ioffe E, Stanley P, : Mice lacking N-acetylglucosaminyltransferase I activity die at mid-gestation, revealing an essential role for complex or hybrid N-linked carbohydrates. Proc. Natl. Acad. Sci. USA 91, 782-732, 1994 |
|
(2) |
Metzler M, et al.: Complex asparagine-linked oligosaccharides are required for morphogenic events during post-implantation development. EMBO J. 13, 2056-2065, 1994 |
|
(3) |
Furukawa K, et al.: Novel function of complex carbohydrates elucidated by the mutant mice of glycosyltransferase genes. BBA 1525, 1-12, 2001 |
|
(4) |
Chui D, et al.: Alpha-mannosidase-II deficiency results in dyserythropoiesis and unveils an alternate pathway in oligosaccharide biosynthesis. Cell 90, 157-167, 1997 |
|
(5) |
Granovsky M, et al.: Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nature Med, 6, 306-312, 2000 |
|
(6) |
Maly P, et al.: The  (1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86, 643-653, 1996 (1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86, 643-653, 1996 |
|
(7) |
Ellies LG, et al.: Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity 9, 881-891, 1998 |
|
(8) |
Bullock SL, et al.: Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev, 12, 1894-1906, 1998 |
|
|
|
|
|
| Jun. 15, 2002 |
|
|
|
|
|
|



