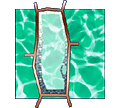
 |
Cellulose-callose syndrome
When we attempt to synthesize cellulose (1,4-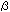 -glucan)
from UDP-glucose with plant plasma membranes, callose is predominantly
formed instead. Although cellulose formed in plants is the most abundant
biopolymer on Earth, predominant formation of callose from UDP-glucose
by the membranes is a major side product (symptom). This is characteristic
of this “syndrome,” which has interested plant biologists ever since the
first attempt was made to synthesize cellulose from UDP-glucose using
plant enzymes. Callose synthase is often used as a marker for the plasma
membrane and is suggested to be an altered form of cellulose synthase.
In fact, callose synthase observed at the end of fibrils forms a complex
with a doughnut-shaped structure, 20 to 30 nm in diameter, which is similar
to the terminal complex for cellulose synthases (1). Recently, studies
of the normalization of cellulose synthesis resulted in successful cellulose
biosynthesis, which was different from callose synthesis. In addition,
the gene for cellulose synthase (CesA) is also different from that
of callose synthase (Csl). -glucan)
from UDP-glucose with plant plasma membranes, callose is predominantly
formed instead. Although cellulose formed in plants is the most abundant
biopolymer on Earth, predominant formation of callose from UDP-glucose
by the membranes is a major side product (symptom). This is characteristic
of this “syndrome,” which has interested plant biologists ever since the
first attempt was made to synthesize cellulose from UDP-glucose using
plant enzymes. Callose synthase is often used as a marker for the plasma
membrane and is suggested to be an altered form of cellulose synthase.
In fact, callose synthase observed at the end of fibrils forms a complex
with a doughnut-shaped structure, 20 to 30 nm in diameter, which is similar
to the terminal complex for cellulose synthases (1). Recently, studies
of the normalization of cellulose synthesis resulted in successful cellulose
biosynthesis, which was different from callose synthesis. In addition,
the gene for cellulose synthase (CesA) is also different from that
of callose synthase (Csl).
Microtubule-callose syndrome
Parallelism of microtubules to callose fibrils and loss of control over
fibril orientation following microtubule disruption with propyzamide are
characteristics of a “syndrome” that was observed in the glucan synthesis
from UDP-glucose in the sheets of the plasma membrane (2). Callose synthase
may bind to microtubules or microtubule-associated proteins directly or
indirectly via other components of the enzyme complex. This syndrome is
related to the fact that cellulose microfibrils are deposited parallel
to cortical microtubules in plants.
Wound response-callose syndrome
Callose deposition induced by physiological stress, chemical and mechanical
wounds, and pathogen infection of plant cells are characteristics of this
“syndrome,” in which callose papillae barriers are believed to physically
protect and impede pathogen attacks on plant cells. Although AtCSL5 (AtCalS12)
is responsible for callose synthase related to wound response, its knockout
mutant did not show a pathogen-induced callose formation but instead gained
a phenotype of resistance to pathogens. Nishimura et al. (3) suggest
that callose could be an induced defense response with negative feedback
regulation by which callose synthesis suppresses the pathway of salicylic
acid-dependent disease resistance. The salicylic- and pathogen-responsive
genes are upregulated in the mutants, and these genes are hyperinduced
after infection. In addition, Jacobs et al. (4) suggest that callose
protects fungi during pathogenesis either by facilitating nutrient uptake
or by preventing host-pathogen interactions.
Of the many problems associated with callose, the major question remains
its actual function in plants. |
|
|
| References |
(1) |
Hayashi T, Read SM, Bussell J, Thelen M, Lin FC, Brown Jr RM,
Delmer DP: UDP-Glucose:(1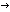 3)- 3)-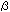 -glucan
synthases from mung bean and cotton. Plant Physiol, 83,
1054-1062, 1987 -glucan
synthases from mung bean and cotton. Plant Physiol, 83,
1054-1062, 1987 |
|
(2) |
Hirai N, Sonobe S, Hayashi T: In situ synthesis of 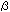 -glucan
microfibrils on tobacco plasma membrane sheets. Proc Natl Acad
Sci USA, 95, 15102-15106, 1998 -glucan
microfibrils on tobacco plasma membrane sheets. Proc Natl Acad
Sci USA, 95, 15102-15106, 1998 |
|
|
(3) |
Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville
SC: Loss of a callose synthase results in salicylic acid-dependent
disease resistance. Science, 301, 969-972, 2003 |
|
|
(4) |
Jacobs AK, Lipka V, Burton RA, Panstruga R, Strizhov N, Schulze-Lefert
P, Fincher GB: An Arabidopsis callose synthase, GSL5, is required
for wound and papillary callose formation. Plant Cell, 15,
2503-2513, 2003 |
|
|
|
|
|



