

 |
 |
Biological Functions of a Soluble Form of N-acetylglucosaminyltransferase V (GnT-V) |
|||||||||||||||||
 |
It is a well-known fact that oligosaccharide structures
of glycoproteins and expression of glycosyltransferases change in malignant
transformation. In approximately 300 kinds of these enzymes, N-acetylglucosaminyltransferase
V (GnT-V) is one of the most important glycosyltransferases which are
involved in tumor metastasis. Recent studies of GnT-V-deficient mice have
demonstrated that GnT-V is essential for tumor metastasis as well as tumor
growth (1). Mammary tumor growth and metastases induced by the polyomavirus
middle T oncogene were considerably less in GnT-V- deficient mice than
in transgenic littermates expressing GnT-V. On the other hand, since expression
of GnT-V was elevated at the stage of chronic hepatitis as well as in
the metastatic lesion of a rodent model of hepatocarcinogenesis (2), GnT-V
is assumed to be involved in the early event of carcinogenesis. The most
important biological phenomenon in the early phase of carcinogenesis is
expected to be immortalization and tumor angiogenesis. In 1993, PierceÍs
and TaniguchiÍs groups performed protein purification and cDNA cloning
of GnT-V. After 10 years, a unique function of GnT-V as an angiogenesis
inducer was found (3). The mechanisms for GnT-V-induced tumor angiogenesis
were due to completely different functions from those of glycosyltransferases.
In general, glycosyltransferases are located in the Golgi apparatus and
involved in the oligosaccharide modification of glycoproteins/glycolipids.
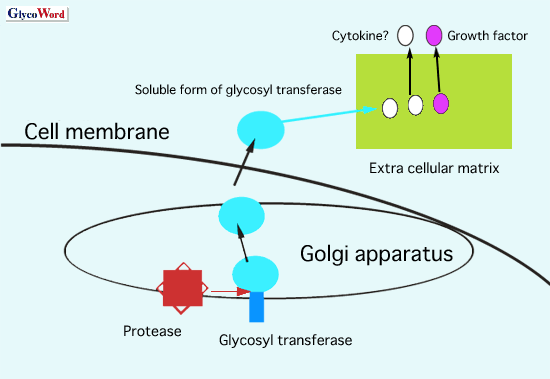
However, a glycosyltransferase also exists in the serum of patients and
it is reported to be a biomarker for certain diseases. Certain proteases
digest a glycosyltransferase and secrete into the condition medium of
cells. This type of glycosyltransferase is the soluble form. In the case
of GnT-V, the soluble form of the enzyme has the biological function of
angiogenesis. The basic domain of GnT-V, KRKRKK, which is located in the
264-269 amino acid sequence, promotes a release of bFGF from the extra
cellular matrix, resulting in tumor angiogenesis (see Fig.1).
Many kinds of cytokines/growth factors attach to proteoglycans such as
heparansulfates in the extra cellular matrix. If the basic domain of GnT-V
is associated with the release or activation of these factors, the soluble
form of GnT-V could have a variety of biological functions. Furthermore,
the identification of a protease which is responsible for cleavage and
secretion of GnT-V may provide new insights in glycobiology. |
||||||||||||||||
 |
|||||||||||||||||
|
|||||||||||||||||
| Eiji Miyoshi (Osaka University, Graduate School of Medicine, Department of Molecular Biochemistry & Clinical Investigation) | |||||||||||||||||
|
|||||||||||||||||
| Nov. 20, 2003 | |||||||||||||||||
|
|
|||||||||||||||||
|
|||||||||||||||||